What Is the Total Number of Atoms in 3na2so4
It is nonflammable. As each molecule contains six carbon atoms 12 H atoms and six O atoms.

How Many Total Atoms Are In 3na2so4 Brainly Com
It is widely used to make soda ash.

. A compound is two or more different atoms that are bonded. How many total atoms in 3 Na2 s O4. Chemistry 23062019 0630.
I assume you were trying to count oxygen atoms for the purpose of balancing an equation and wanted. What is 2 21 14 10 2. Is the correct coefficients for this balanced equation C7H10N O2 CO2 H2O NO2.
A B -- AB is the skeletal equation. How many atoms are in 4ca3 po42. Describe how minerals form.
There are 4 oxygen atoms in each phosphate PO 4 3- ion and two phosphate ions in each calcium phosphate formula unit for a total of 428 oxygen atoms per formula unitFour formula units of calcium phosphate have 4832 atoms of oxygen present. Get the Atomic Weights of Each Element Find th. How many total atoms are in 3Na2SO4.
Get the Chemical Formula Get the chemical formula of the compound. C total hydrogen atoms are present 312 36 H atoms. How do you calculate the number of atoms in a mole.
Avogadros number tells me the amount. How many total atoms are in 3na2so4. Multiply the coefficient times the subscript for each element.
Which type of reaction can be recognized by the general pattern ABX-AXB. The 3 is a coefficient or subscript HELPPPP Can someone help me please. 1 Get Other questions on the subject.
How many total atoms are in 3Na2SO4. How do you calculate the number of atoms. The 3 is a coefficient or subscript HEL.
Thus O2 is only a molecule because it is more that one atom and all atoms of the molecule are the same. Total of atoms. Answer 1 of 2.
The graph shows the number of gallons of white paint that were mixed with the gallons of green paint in various different ratios. For example the molar mass of phosphorus is 30974 gramsmole. To convert from moles to atoms multiply the molar amount by Avogadros number.
How many total atoms are in 4Ca3PO42. Sodium Sulfate comes in a few different forms like Heptahydrate sodium sulfate but this is not commonly seen. To calculate the number of atoms in a sample divide its weight in grams by the amu atomic mass from the periodic table then multiply the result by Avogadros number.
The products of Cu FeNO 3 2. An unknown compound is found to burn in oxygen. Five atoms within the parentheses x three 15 two aluminums give a total of 17.
Multiply the coefficient times the subscript for each element. How are exact numbers treated differently from other numbers in a calculation. Which sample at stp has the same number of atoms as 18 liters of ne at stp.
1 mole 60221023 6022 10 23 atoms molecules protons etc. For example if the compound is sodium sulfate Na2SO4 each molecule contains two atoms of sodium Na one atom of sulfur S and four atoms of oxygen O. You will note that the SO43 means that the number of atoms bound by the parentheses have one sulfur and four oxygens.
Lets count the number of atoms in one formula unit. How many total atoms are in 3Na2SO4. Correct answer - 5 3Na2SO4 of molecules.
Total of atoms. Sodium sulfate is also called Glaubers salt. For 3Na2SO4 there are a total of 6 sodium atoms 3 sulfur atomsand 12 oxygen atoms.
Chemistry 22062019 1200 daytonalive83481. A molecule is two or more atoms bonded together that may or may not be the same element. To convert from atoms to moles divide the atom amount by Avogadros number or multiply by its reciprocal.
Avogadros number is a very important relationship to remember. What is an exact number. For 3Na2SO4 there are a total of 6.
3Na2SO4 has 21 atoms in it based on the numbers that are before and after the element in it. Bleach is made up of one sodium atom one chlorine atom and one oxygen atom. 5 3Na2SO4 of molecules.
Is the total number of atoms in 3Na2SO4. The three to the right means that all the atoms within the parentheses are multiplied by three. Ican determine the molar mass of an element by looking on the under the atomic mass for the element.
How many molecules in. To convert from atoms to moles divide the atom amount by Avogadros number or multiply by its reciprocal. How many total atoms are in 3Na2SO4.
So it works like this.
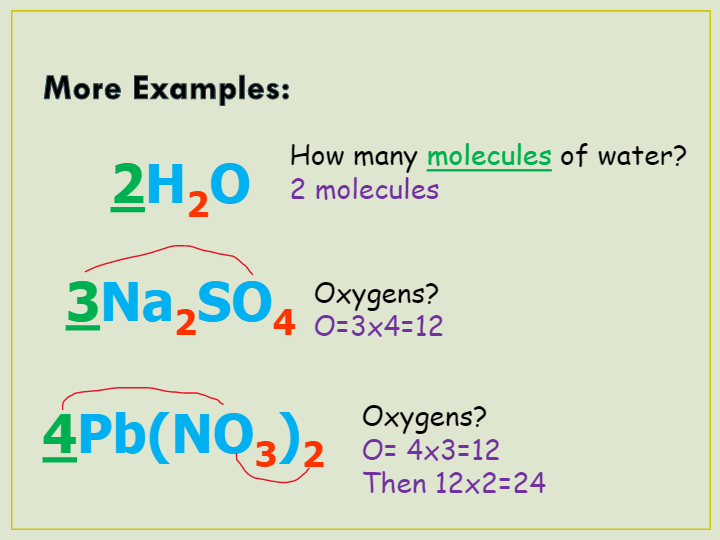
Chemical Equations Counting Atoms Tek 7c Quizizz

How To Find The Number Of Atoms In Na2so4 Sodium Sulfate Youtube

Red Station Determine The Total Number Of Atoms In Each Formula 1 H2so4 2 3fe203 3 Agno3 4 Pb No3 2 5 Ba Clo3 2 7 15 5 9 8 Ppt Download
No comments for "What Is the Total Number of Atoms in 3na2so4"
Post a Comment